When DOE originally applied for approval from EPA, DOE made several assumptions about plutonium solubility that were not correct. They did not have actual data, because the tests that would provide this data had not been completed; nevertheless, they applied for and received approval for WIPP. They assumed that the plutonium would only exist in less soluble oxidation states than it actually will. They compounded this error by using thorium solubility data in their calculations instead of data for plutonium. We now know that thorium solubility is not at all representative of plutonium or uranium solubility under WIPP conditions. In fact, in one instance, uranium was shown to be 10,000 times more soluble than thorium. DOE also did not include the effects of all the chelating agents on plutonium solubility in their application. The Actinide Source-Term Waste Test Program at Los Alamos has shown that in some instances the presence of these chemicals increase the solubility of plutonium by a factor of 1000. Finally, DOE used a formula for calculating solubilities that is unique to WIPP and predicts solubilities that "...can not be explained by chemistry, thus leaving the reliability of the calculations suspect." (EEG-77: Plutonium Chemistry Under Conditions Relevant for WIPP Performance Assessment)
Many of the gases (hydrogen, methane and oxygen) are flammable. Most of the waste is packed in plastic bags which can create a static electrical spark when moved. Falling rockbolts or drums could also cause a spark. The DOE has said that "...there is a reasonable potential for collections of flammable and possibly detonable mixtures of hydrogen, methane and oxygen in the room headspace above the backfilled waste after emplacement of the composite panel seal." (Memo on: Potential for and possible Impacts of Generation of Flammable and/or Detonable Gas Mixtures During the WIPP Transportation, Test, and Operational Phases, Slezak and Lappin, Sandia National Laboratories, 1990)
This combination of explosive gases and ignition sources creates the potential for fire or explosion in the waste rooms. In addition, plutonium and uranium are pyrophoric which means that under certain conditions they can spontaneous ignite in the presence of oxygen. Although the panel plugs are robust, DOE can only theorize that they will hold during an explosion; no actual tests under WIPP conditions have ever been done. Because of the amount of radioactivity involved, there would be no way to fight these radioactive and hazardous waste fires once they started. Unless the fires went out by themselves from lack of oxygen or were smothered by roof-falls, they could smolder on indefinitely.
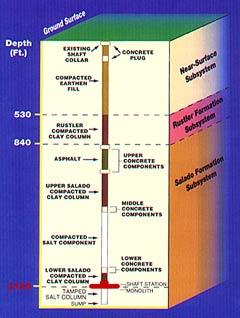
The plugs for the WIPP shafts are extremely massive and consist of various layers of both local natural materials like excavated Salado salt, and artificial materials like grout, cement and asphalt. However, like the panel closures, the technology has never been tested under conditions that will exist at WIPP after it is closed. The ability of the shaft plugs to contain the waste is only theoretical. Also, at the same time that the 2014 release occurred, DOE was trying to obtain reduced closure requirements so the panel closures could be less robust
Salt will cause corrosion of the sealing materials in the shafts as it does in the boreholes around WIPP. A small leak along the contact between the walls of the shafts and the shaft fill will soon become enlarged into a solution passage. (After closure, the water that is flowing into the Exhaust Shaft will seek the easiest path, which could be down this interface between the plugs and the walls of the shafts.) In the end, however, sealing the shafts and nearby boreholes is immaterial if subsidence fractures breach the plugs.
The panel closures, as described above, are also fairly massive but again, are only theoretically able to withstand an explosion or fire in a panel, a roof fall nearby or the effects of salt creep. CARD and other groups had asked since at least the late 80s for the panel closure designs (and the shaft seal designs) to be tested. DOE had always refused and both EPA and the New Mexico Environment Department went along with this. When they finally began testing their ability to implement the panel closure design, they found that they were unable to meet required specifications. Pouring the concrete into huge forms in theunderground also turned out to be extremely difficult. As DOE said, "[T]esting of the Salado Mass Concrete (SMC) formulation specified...has highlighted uncertainties relative to attaining the required target values of strength and workability, and thus, construction of the monolith portion of the approved...Panel Closure design is problematic." DOE then proposed another panel closure design which would consist of a 30' explosion wall, no isolation zone, and 100' of backfilled salt. Again, this design had never been tested and its ability to provide safety was only theoretical.
and both EPA and the New Mexico Environment Department went along with this. When they finally began testing their ability to implement the panel closure design, they found that they were unable to meet required specifications. Pouring the concrete into huge forms in theunderground also turned out to be extremely difficult. As DOE said, "[T]esting of the Salado Mass Concrete (SMC) formulation specified...has highlighted uncertainties relative to attaining the required target values of strength and workability, and thus, construction of the monolith portion of the approved...Panel Closure design is problematic." DOE then proposed another panel closure design which would consist of a 30' explosion wall, no isolation zone, and 100' of backfilled salt. Again, this design had never been tested and its ability to provide safety was only theoretical.
The panel closures are not seals so air and gases can move in and out to some extent, although most particles cannot. The room closures are not at all robust and are constructed primarily to control the movement of the repository air and direct airflow into active rooms. They also are not seals but are supposed to keep waste from getting into active areas of the repository. However, it is extremely questionable if these barriers could keep waste—especially powdered waste—from reaching the rest of the repository if there were a roof-fall in a closed room that was in a still active panel. A large roof-fall could crush the waste containers, releasing waste and could destroy the room barrier if the fall were near it. A large roof-fall could also create such a large pressure wave that it could flatten one or more barriers and push material into active areas. (Dr. David Snow reported once being almost knocked over by the pressure of a roof-fall that occurred about a mile away from where he was standing.)
By 1990 most of the underground had been bolted together with hundreds of 6- to 10-foot-long rock bolts, rebar and wire mesh. Some rooms in Panel One had a second and even a third roof support system to keep the separated salt from crashing down. Ceiling falls had already occurred in the northern experimental area before the repository opened. Several falls were slabs weighing around 1,500 tons. Each of the hundreds of roofbolts in the ceilings of Rooms 1 and 2 of Panel 1 had to be "detensioned" about once a month and the walls and floors of the repository had to be periodically milled since they were bulging and cracking. In addition, as the pressure from the creeping salt broke the rockbolts, they had to be replaced throughout the underground. Not an ideal situation for waste emplacement.
 This necessary maintenance cannot be performed after wastes have been emplaced in a room since access to the ceiling is blocked by the waste. Without maintenance, the likelihood of a roof fall increases. In addition to the safety problems, all the metal holding the repository together, along with the metal 55-gallon drums in which the waste is packed are another source for hydrogen gas generation
This necessary maintenance cannot be performed after wastes have been emplaced in a room since access to the ceiling is blocked by the waste. Without maintenance, the likelihood of a roof fall increases. In addition to the safety problems, all the metal holding the repository together, along with the metal 55-gallon drums in which the waste is packed are another source for hydrogen gas generation
If records on current activities are not given any attention, how likely are they to be looked at 100 or 1000 years from now? And would anyone 1000 years from now even be able to read any records that might have survived? How many people today can understand the English of 1000 years ago? Try this old English sentence from Beowolf: Bealo-cwealm hafao felu fork-cynna foro onsended. It means: Death-qualm has sent full many a folk forth on their way.